Let's start of with the structure of graphite. Graphite has a unique hexangonal structure which is a result of the carbon atoms forming 3 bonds with each other (as opposed to 4 bonds normally).
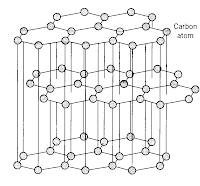 As you can see from the diagram, each carbon is only bonded to 3 other atoms and they are arranged in hexangonal layers and the layers are held together by weak intermolecular force of attraction (or van de waal's forces).
As you can see from the diagram, each carbon is only bonded to 3 other atoms and they are arranged in hexangonal layers and the layers are held together by weak intermolecular force of attraction (or van de waal's forces).Lets look at diamond now. Carbon atoms in diamond bonded to 4 other carbon atoms in a tetrahedral structure. This rigid and tough structure gives diamond its ultra tough properties, like hardness, high melting boiling points.
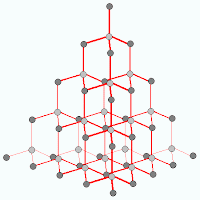
For comparison of properties regarding graphite and diamond stay tune!
Enjoy!
Entry by
Json Lim
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.