Wednesday, September 30, 2009
Redox
Definition: reactions which both oxidation and reduction reactions take place at the same time.
Oxidation
Gain in oxygen
Lose of electrons
Lose of hydrogen
Increase in oxidation state number
Reduction
Lose of oxygen
Gain of electrons
Gain in hydrogen
Decrease in oxidation state number
Fixed Oxidation numbers
0
All elements, noble gases, metals
+1
Group 1 ions, H+
+2
Group 2 ions
+3
Al3+
-1
Group 7 ions, Oxygen in H2O2, hydrogen in Metal hydrides e.g NaH
-2
O2-, S2-.
-3
Nitrides N3-
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Rate of Reaction
Rate of reaction can be calculated and compare by measuring a loss of mass or amount of gas collected.
Factors affected rate of reaction
• Particle size
• Temperature
• Pressure (only for all gas system)
• Catalyst
• Concentration
Use this standard statement to answer your question.
Increase in temperature leads to an increase in the energy of the particles which leads to an increase in number of collisions. This leads to an increase in number of effective collisions which leads to an increase in rate of reaction. The Itallic and bold words can be change to accommodate the factors.
e.g. Increase in particle sizes leads to an increase in the surface area of the reagent which leads to an increase in number of collisions. This leads to an increase in rate of reaction which leads to an increase in rate of reaction. GOT IT? When plotting graphs, the number of moles of products determine the end point of the reaction!
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Energy Changes
Enthalpy change is the amount of heat released or absorbed when a chemical reaction occurs.
Exothermic reaction – liberates heat energy to the surroundings which results in a general increase in the temperature of the surroundings.
Endothermic reaction – absorbs heat energy to the surroundings which results in a general decrease in the temperature of the surroundings.
Type of reactions Enthalpy Product energy level
Exothermic
ΔH= - ve because more heat is given out during bond forming than heat taken in to break them
Product is at a lower energy compared to reagent.
Bonds of products are stronger than bonds in reagent.
Endothermic
ΔH=+ ve because more heat is taken in to break bonds than heat is given out when bonds form.
Product is at a higher energy compared to reagent.
Bonds of reactants are stronger than products.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Electrolytes
• Molten salt – the 2 ions will discharge
• Aqueous salt – hydrogen (unless metal is below hydrogen in that case the metal will discharge) and hydroxide ions (ALWAYS) will discharge
• Acidic solution - hydrogen and hydroxide ions will discharge
• Concentrated chlorides solution – ALWAYS chloride ions, positive ions the usual method of looking at reactivity series of methods for discharge order.
• Reactive anode (used in the purification of copper or electroplating) – anode dissolves into the solution. UNDER NO CIRCUMSTANCES will cathode dissolve. Even in simple cells the plate that dissolve (i.e. the more reactive plate that dissolves in the anode).
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Intermolecular Forces and Intramolecular forces for Graphite
When explaining the high temperature in which graphite melts, we say that they are breaking strong covalent bonds. (Pure)
When explaining why graphite act like a lubricant, we say that the graphite molecules are arranged in layers which are held by weak IMF therefore can slide over each other easily. GET IT? (Pure)
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Bonding
Chemical bond is a force of attraction holding atoms together in a molecule or crystal
Three types of chemical bonds
• Ionic bonding
• Covalent bonding
• Metallic bonding
Ionic bonding – involving metal and non-metal elements. Force of attraction between oppositely charged ions. Only exception is compounds like ammonium salts. Although ammonium is made up entirely of NON_METAL, it is still an Ionic compound because it contains charges.
Covalent bond – formed between 2 combining atoms of non-metallic by mutual sharing of one or more electrons
Metallic bonding – type of chemical bond that holds the atoms together in a solid metal. It’s the attraction between the sea of electrons and the positive metal ions. (more important for pure chem.)
Covalent compounds
- simple molecular compounds like hydrogen molecule, water molecule etc.
- giant molecular structure like diamonds and silicon dioxide (pure)
- graphite
Ionic compounds – self explanatory
Metallic substances – pure metals and alloys
Types of substances
Simple molecular
Types of bonds broken during change of state: Intermolecular forces of attraction.
Strength of the bond broken:Weak
Melting point and Boiling point:Low
Giant molecular or Giant Covalent
Types of bonds broken during change of state:Covalent
Strength of the bond broken:Strong
Melting point and Boiling point:High
Ionic Compounds
Types of bonds broken during change of state:Ionic Bonds
Strength of the bond broken:Strong
Melting point and Boiling point:High
Metals Types of bonds broken during change of state:Metallic Bonds
Strength of the bond broken:Strong
Melting point and Boiling point:High
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Atomic Structure Again
Particle
Proton
Relative mass 1
Relative Charge +1
Neutron
Relative mass 1
Relative Charge 0
Electron
Relative mass 1/2000 or appro = 0 or 1/1840
Relative Charge -1
Proton number = Number of protons or neutrons in an atom
Nucleon number = sum of number of neutrons and protons in an atom. (Note this is not the same as relative atomic mass although in our syllabus it is the same for most atoms)
Isotopes are atoms of the same element having the same atomic number but different number of neutrons
Remember anything more than proton number 20, you CANNOT draw the electronic configuration. If the questions or number of valence electrons, LOOK AT GROUP NUMBER!!
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Test For Gases
Gases
Ammonia
Colorless, pungent
Turns Red litmus blue
Carbon dioxide
Colorless, Odourless
Gives a white ppt with limewater
Chlorine
Greenish Yellow, pungent
Turns Blue litmus red and then bleaches red litmus
Hydrogen
Colorless, Odourless
Extinguish lighted splint with a pop sound
Oxygen
Colorless, Odourless
Relights glowing splint
Sulphur dioxide
Colorless, Choking
Turns acidified potassium dichromate from orange to green
Nitrogen dioxide
Reddish Brown, pungent
Turns Blue litmus red
Water
Colorless Odourless
Condenses into cold water. Turns White anhydrous copper sulphate turns blue,
Cobalt chloride paper turns blue to pink
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Purification
Separation Techniques
Method
Filtration
Remove insoluble substances from liquids
Distillation
Obtain solvent from solute (water from salt water)
Fractional Distillation
Separate a mixture of miscible liquids (petroleum, ethanol and water)
Separating funnel
Separate a mixture of immiscible liquids (oil and water)
Crystallization
Obtain solute from solution (sugar from sugar solution, salts from their own salt solution)
Chromatography
Separate mixtures that contain chemically similar components. Very often used for identification
Crystallization (important for salt making)
Steps to take
• Heat the solution to obtain a saturated solution
• Once saturated, allow the solution to cool
• Crystals of the salt will appear
• Filter the crystals from the solution
• Dry the salt between filter paper
Chromatography (Pure Chemistry only)
• Rf value = distance moved by the spot/distance move by the solvent front. Rf value cannot be more than 1.
Test for purity
• Test for constant melting and boiling point
• Chromatography
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Equipment to measure volumes
Apparatus
Stopwatches
Seconds, time
Thermometer
Degrees Celsius, temperature
Beaker
Cm3, rough volume up to 250.
Measuring cylinder
Cm3, volume nearest to Cm3
Pipette
Cm3, fixed volume of 5, 10, 20, 25, 50 Cm3
Burette
Cm3, volume nearest o.1 Cm3
Gas syringe
Cm3, volume of gas
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Terms used in Science Examinations
• Define: you are only required to write a concise statement to say what something is or means
• State: A short, concise answer is expected without explanation
• Explain: Means some reference to Chemical theory
• Describe: You are to state the main points in words (sometimes with diagram, if appropriate)
• Discuss: You are to give a critical account of the points in a topic
• Predict or Deduce: You are to deduce an answer from information in the question or from earlier answers (you are not expected to produce answer from memory)
• Suggest: You are not expected to know the correct answer. You are to make a logical deduction from the information given in the question or passage.
• Comment: Give points of interest which are relevant to the question. There can be many answers and the number points you give depends on the marks for the question.
• Find: Normally calculate, measure etc
• Determine: answers normally obtained by calculations or reading of a graph
• Estimate: obtain an answer as accurately as possible.
• Construct: Normally applies to the construct of the chemical equations
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Tuesday, September 22, 2009
Tuesday, August 11, 2009
Graphite vs Diamond Properties - Their structure
Let's start of with the structure of graphite. Graphite has a unique hexangonal structure which is a result of the carbon atoms forming 3 bonds with each other (as opposed to 4 bonds normally).
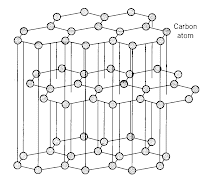 As you can see from the diagram, each carbon is only bonded to 3 other atoms and they are arranged in hexangonal layers and the layers are held together by weak intermolecular force of attraction (or van de waal's forces).
As you can see from the diagram, each carbon is only bonded to 3 other atoms and they are arranged in hexangonal layers and the layers are held together by weak intermolecular force of attraction (or van de waal's forces).Lets look at diamond now. Carbon atoms in diamond bonded to 4 other carbon atoms in a tetrahedral structure. This rigid and tough structure gives diamond its ultra tough properties, like hardness, high melting boiling points.
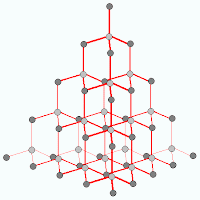
For comparison of properties regarding graphite and diamond stay tune!
Enjoy!
Entry by
Json Lim
Monday, August 10, 2009
Titration Salt Making Procedures
a)The reactants you are using to produce them,
b)The chemical equation,
c)Write up steps to produce the salt.
This blog entry is for titration, hence the first thing to decide is the reactants needed reaction.
A) Reactants
i)An Acid - Well you know the usual.
ii)An Alkali - To identify an alkalis, they are any group 1 hydroxides, ammonium hydroxides and calcium hydroxide.
That's all.
Let's take this salt as an example, sodium sulfate.
As the name of the salt suggest, the sulfate part comes from the acid used and the sodium part comes from the alkali.
Logically these are the reactants,
Acid - sulfuric acid (since the salt is a sulfate)
Alkali - sodium hydroxide (since the salt is a sodium substance)
So there is it, the reactants.
Next the chemical equation.
Since Alkali + Acid -------> Salt + Water
We will have this word equation
Sodium hydroxide + sulfuric acid --------> Sodium sulfate + water
2NaOH(aq) + H2SO4(aq) ------> Na2SO4(aq) + 2H2O(l)
Last part is the write up. This is the template we can use.
1. Pipette 25 cm3 of acid into a conical flask. Add 2-3 drops of a suitable indicator.
2. Add alkali from a burette gradually until the indicator changes colour. Note the volume of the alkali needed to neutralise the acid.
3. Repeat the titration by adding this volume of alkali to another 25 cm3 of acid.
4. Evaporate the salt solution until it becomes saturated.
5. Filter the crystals and dry between filter paper.
So in our answer we change the keywords to fit our reagents, so this is how our eassay would look like. Of course the eassay will not be in point form, i put it this way to aid understanding. The keywords that i changed are in red.
1. Pipette 25 cm3 of sulfuric acid into a conical flask. Add 2-3 drops of a suitable indicator.
2. Add sodium hydroxide from a burette gradually until the indicator changes colour. Note the volume of the sodium hydroxide needed to neutralise the sulfuric acid.
3. Repeat the titration by adding this volume of sodium hydroxide to another 25 cm3 of sulfuric acid.
4. Evaporate the sodium sulfate solution until it becomes saturated.
5. Filter the sodium sulfate crystals formed and dry between filter paper.
There you go, this method is salt making for titration.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Salt Making - Selection of Method
a)Titration
b)Precipitation
c)Acid Reactions (In my tuition class, we call this excess reagent method)
The selection technique is very simple. Simply ask yourself if the salt is soluble. This should ALWAYS be the first thing you ask yourself.
If the salt is soluble, use either titration or acid reactions.
For titration, it is for salts that contain the following ions: Group 1 ions and Ammonium ion.
E.g. sodium chloride, potassium nitrate, ammonium sulfate, lithium ethanoate.
For acid reactions, it is for all other salts that are soluble.
E.g. copper (II) sulfate, magnesium chloride, barium nitrate, silver nitrate.
That's all for the selection technique for soluble salts.
If the salt is insoluble, use precipitation. That's the only method.
E.g. barium sulfate, lead (II) sulfate, lead(II) chloride, silver chloride, calcium sulfate. Notice no nitrate salts here since all nitrates are soluble.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Comparing the MP/BP of Ionic and Covalent Compouds

For our answer, it will be definitely be the ionic compound of magnesium oxide. For those who are lost, this is because ionic compounds have high MP and BP while simple covalent compounds have low MP and BP. If you have no clue how to differentiate between ionic and covalent compounds refer to this post.
Now the next step is to explain why does magnesium oxide has high MP and BP.
This is the suggested template.
a)Start by identify the type of compounds for the substances.
For our question magnesium oxide is the ionic compound and the oxide of phophorous is a simple covalent molecule.
b) Next, tell the marker the kinds of bonds broken when the substances undergo change of state.
This is the general rule of selection when it comes to identifying the kinds of bond broken.
Ionic Compounds --- break strong ionic bonds during change of state (aka melting and boiling)
Giant Covalent Compounds --- break strong covalent bonds during change of state (aka melting and boiling) Simple Covalent Molecules --- break weak intermolecular force of attraction or van da waal's forces of attraction during change of state (aka melting and boiling)
c)Last part sum up your answers by telling the marker the amount of energy needed to carry out the change of state.
That's all. I am gonna end this post by writing a sample answer for this type of question.
Qn: Which of the 2 substances, magnesium oxide and phophorous oxide has a higher melting point. Explain.
Ans: Magnesium oxide is an ionic compound and phosphorous oxide is a simple covalent compound. During change of state, magenesium oxide breaks strong ionic bonds while phosphorous oxide breaks weak intermolecular force of attraction between the molecules. Hence a lot of energy is needed to break the strong ionic bonds therefore MP is high, while lesser energy is needed to break the weak intermolecular force of attraction hence MP of simple covalent molescules is low.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Tuesday, August 4, 2009
Acid Reactions Part 1
a)nitric acids
b)sulfuric acids
c)nitric acids
d)ethanoic acids
Acids react with a 4 main substances for our syllabus, namely,
a)metals
b)metal oxide
c)metal hydroxide
d)metal carbonate
Below is the flow chart you are expected to observed when an acid reacts with the above substances.

Enjoy!
http://www.oleveltuition.com/
Monday, August 3, 2009
Atomic Structure - The basics
a)nucleus
b)electron shells.
The Nucleus
Simply put it, it is the part of the atom which you will find 2 kinds of particles, namely protons and neutrons.
Protons
Positively charged particle and it has a relative mass unit of 1.
Neutrons
Neutral and uncharged particle and it has a relative mass unit of 1.
Ok that basically sums up the particles found in the nucleus, lets go on to the electron shells.
Electron shells
Well as the name suggests, electrons are found here. Basically the electron shells are like holla hoops around a person and the electrons found in the electron shells spinning around the person at great speed. In an atom, the electron shells are found outside the nucleus and the electrons are spinning around the nucleus
Electrons
Negatively charged particles and have negligible mass compared to the other 2 particles.
A quick recap,
Subatomtic Particles Relative charge Relative mass Location
Proton +1 1 In the nucleus
Neutron 0 1 In the nucleus
Electrons -1 0 or 1/1840 Electron shells outside
nucleus
Cheers
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Types of Oxide
Meaning
The compound CaO, is considered an oxide since it is made up of calcium element and oxygen only. While NaOH, sodium hydroxide is not an oxide as it contains 3 elements. Hence in order to be an oxide it has to follow the sequence below.
Element A + Oxygen -----> A Oxide.
There are 2 major categories of oxide, namely metallic and non metallic oxide.
Metallic Oxide
Basic Oxide
a)The first type of metallic oxide is known as basic oxide. This group of oxide reacts with acid in a neutralization reaction and if it dissolves in water, it will form alkali (the soluble basic oxides are all the group 1 oxides and calcium hydroxide based on O levels syllabus).
Amphoteric Oxides
b)The second type of metal oxide is made up of zinc oxide, aluminium oxide and lead (II) oxide.
This group of oxides react with both acid and alkalis.
Non Metallic oxides
c)Acidic oxides
Acidic oxides are non metal oxides that produces hydrogen ions when dissolved in water. Otherwise, any non-metal oxides can be classified as acidic oxides. However there are a few exceptions and these are the neutral oxides
d) Neutral oxides
This last group of oxide are made up of all monoxides (carbon monoxide or nitrogen monoxide etc) and water. They do not have an effect on any indicators. Cheers.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/
Formulae of Ions Part 1 - First Twenty Elements
Technique.
Lets select an element, e.g. Magnesium.
Magnesium has the following information based on the periodic table.
Number of protons: 12
Number of neutrons: 12
Number of electrons: 12
The electronic configuration of magnesium is 2,8,2.
The next thing is to ask yourself, in order to obtain a stable electronic configuration (full outer electron shell), the atom needs to do something.
a) Lose 2 electrons so that the configuration becomes 2,8 (full outer electron shell)
b) Gain 6 electrons so that the configuration becomes 2,8,8 (full outer electron shell again)
From all the atoms' point of view, it is easier to lose 2 electrons than to gain 6. So in order to form a stable electronic configuration, magnesium will choose to lose 2 electrons and the updated sub atomic particles looks like this.
Number of protons: 12
Number of neutrons: 12
Number of electrons: 10
Since electrons are negatively charged and protons are positively charged, there will be a total of 12 positive charges and 10 negative charges and hence the overall charge is +2.
Therefore the charge on magnesium is +2
Lets take another example, oxygen.
Subatomic particles of oxygen atom looks like this
Number of protons: 8
Number of neutrons: 8
Number of electrons: 8
Electronic configuration: 2,6
In order to be stable oxygen needs to gain 2 electrons (instead of losing 6 electrons) to form a stable electronic configuration.
After gaining 2 electrons, the new numbner of subatomic particles looks like this.
Number of protons: 8
Number of neutrons: 8
Number of electrons: 10
In this ion, there are 8 positive charges and 10 negative charges, hence it has an overall charge of -2. Therefore the ion of oxygen has a charge of -2.
This is how you find the ionic charge of the first 20 elements. Cheers.
Enjoy!
Entry by
Json Lim
www.oleveltuition.com
Differentiating Ionic and Covalent Compounds
The general rule is basically very simple and i am sure your teachers have mentioned many times.
Ionic compounds - formed by substances that is made up with positive and negative ions.
The EASIEST method to decide if a substance is ionic basically is to look at the formula of the compound. For example, magnesium chloride, basically made up of magnesium ion (metallic) and chloride ion (non-metallic). So to recognize an ionic compound, look out for a metal and a non-metal in formula. This however is just a general guide as there are exception cases.
For example, a compound like ammonium chloride, NH4Cl. Notice there are no metals, in ammonium chloride but it is still considered an ionic compound. Reason is because it is made up of the positive ammonium ion and negative chloride ion. However, it is still quite safe to to identify ionic by the metal - non metal rule.
Finally for covalent substances, its the easiest to identify. As long as the substance does not contain any metals it can be taken to be a covalent substance. That of course taking into account the annoying ammonium based substances which defy this rule.
Enjoy!
Entry by
Json Lim
http://www.oleveltuition.com/